T is not energy. In the above examples we have seen that heat transfer takes place from the hot tea to the Surrounding and also from the surrounding to the ice.

Difference Between Heat And Temperature Comparison Measurement
To understand it clearly in scientific terms heat is the overall energy of the motion of the molecules while temperature is the average energy of the molecular motion.
. Scientifically heat is a form of energy transferred from a hot body to a cold body. Heat unlike temperature is the energy associated with the motion of particles in matter. Heat is basically a kind of energy while temperature is a measure of that energy.
Fahrenheit is the most common scale for day-to-day use in the United States. Temperature is a physical intrinsic characteristic. For example 10 litres of water at 50C will contain much more heat energy than 10 litre of water at 80C even though its temperature is higher.
Heat is measured in Joules J or calories cal while the temperature is measured in Kelvin degrees Celsius or degrees Fahrenheit. Meanwhile temperature is an objective measure of the heat energy contained in a body. On the other hand temperature measurements are in degrees Celsius C.
Fahrenheit Celsius and Kelvin. Heat always transfers from the Hot to the Cold substance and the greater the Temperature Difference. Heat on the other hand is a form of energy that can vary with sample size.
Heat is the process of the transfer of energy from one body to another. It relates to the average kinetic energy of microscopic motions of a single particle in the system per degree of freedom. The main difference between heat and temperature is heat is a form of energy whereas temperature is the measure of energy.
Heat is measured in Joules. Distinguish between heat and temperature. It is also often used as a subjective measure of the heat energy contained in a body.
Heat is a form of energy transferred between two or more systems or a system and surroundings by a virtue of temperature difference. Temperature or the hotness or coldness of an item is measured on three scales. Heat is then communicated via temperature.
Though in casual usage they are often confused heat and temperature are not the same. Weather forecasts food recipes and body temperature are almost always communicated in those terms. In this laboratory activity learners explore the difference between heat and temperature and explore the rate of heat transfer from one substance to another as it depends on the density of the substances being investigated.
The two main types of energy kinetic energy and potential energy are included in the heat energy contained by the molecules in a matter. Actually heat is a form of. While heat is the amount of energy in a body the temperature is the measure of the intensity of the heat.
Heat measurements are in Joules J. The transfer of heat energy is from a higher-temperature material to a lower-temperature substance. The main difference between heat and temperature is that heat is the form of energy stored in the body to perform work while the temperature is the measure of the heat of the body.
Heat is the energy introduced to a body and is a measure of all the energy the body has while temperature is a measure of the kinetic energy of the molecules of the body only. Temperature on the other hand is the degree of coldness or hotness of a body. It is due to the fact that faster-vibrating molecules shift their energy to slower-vibrating molecules.
Heat is measured by calorimeter and. This can be explained with an example. First and foremost heat is categorized as a form of energy whereas temperature is cold or hot.
The terms are extensive due to their wide usage in our daily life. As against temperature measurement is associated with how fast or slow the molecules are moving with the change in heat. Heat is a type of energy that measures the total kinetic energy of the molecules in a body while the temperature is the state of being hot or cold of matter.
Simply put particles in matter move faster if the temperature is higher and slower if the temperature is lower. The flow of energy from a warm to a cooler object is known as heat. It is one of two activities supporting the.
They may sound similar but are much different according to science and research. The differences between heat and temperature are. The difference between heat and temperature is small but significant.
6 rows Heat and temperature are not the same thing. Heat is the molecular motions overall energy while the temperature is. Heat is a physical quantity that describes the energy that passes from a warmer body to a.
9 rows The SI unit Heat is Joule and Temperature is Kelvin. There is a fine line that separates heat from temperature in the sensation that heat is considered a form of energy though temperature is a measure of energy. Heat is the thermal energy transferred between two or more bodies while the temperature is a thermodynamic property that measures the thermal state of a body.
Temperature is an intensive property while heat is an extensive property. The activity can be conducted either in a science lab or in a kitchen. The bodys heat content determines whether it is hot or cold.
Aside from the rest this is perhaps the main difference between heat and temperature. Heat is measured as the product of a number of particles and the energy content of each particle. It is the total amount of energy both kinetic and potential possessed by the molecules in a piece of matter.
Heat measures the total energy both kinetic.
Difference Between Heat And Thermal Energy Difference Between
Difference Between Heat And Temperature With Comparison Chart Key Differences
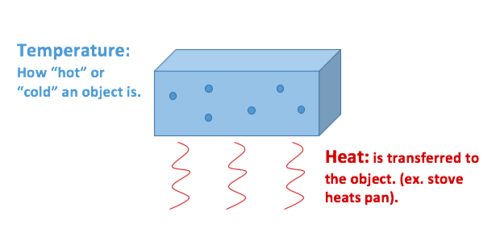
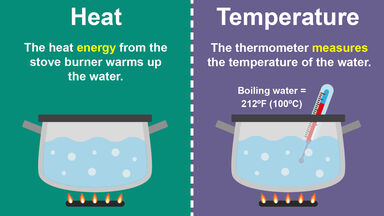

0 Comments